prion news
Open Positions
prion news
Open Positions
| Prion Protein |
Alzheimer's
Disease |
|
 |
|
|
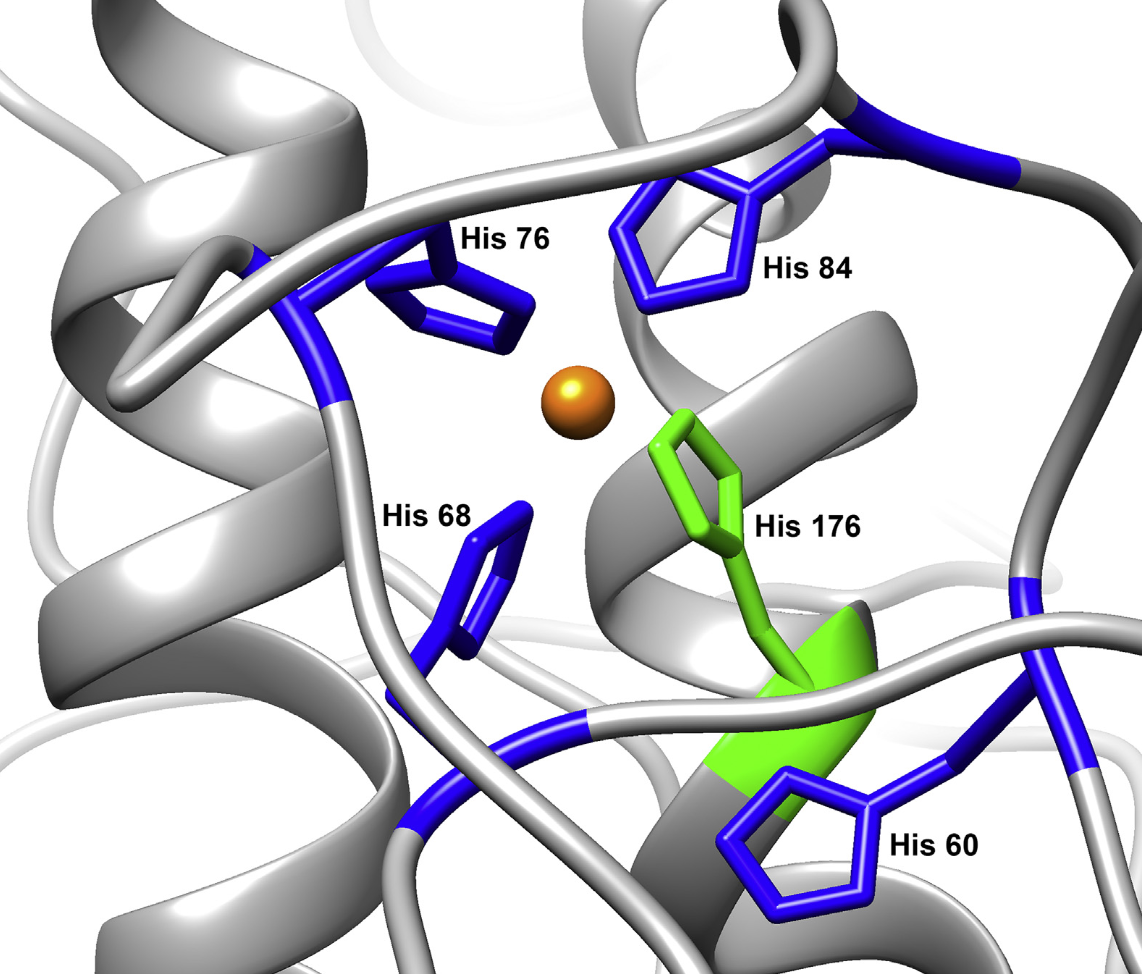
| Misfolding of PrPC
causes prion diseases, which include
kuru, CJD and mad cow disease.
PrP's normal function is related to
copper and zinc regulation in the
central nervous system. Cu2+
promotes an interaction between the
protein's domains that suppresses
inherent neurotoxicity. (J.
Mol. Biol. 432:4408-25 2020,
Meth.
Enzymol. 666:297-314 2022) |
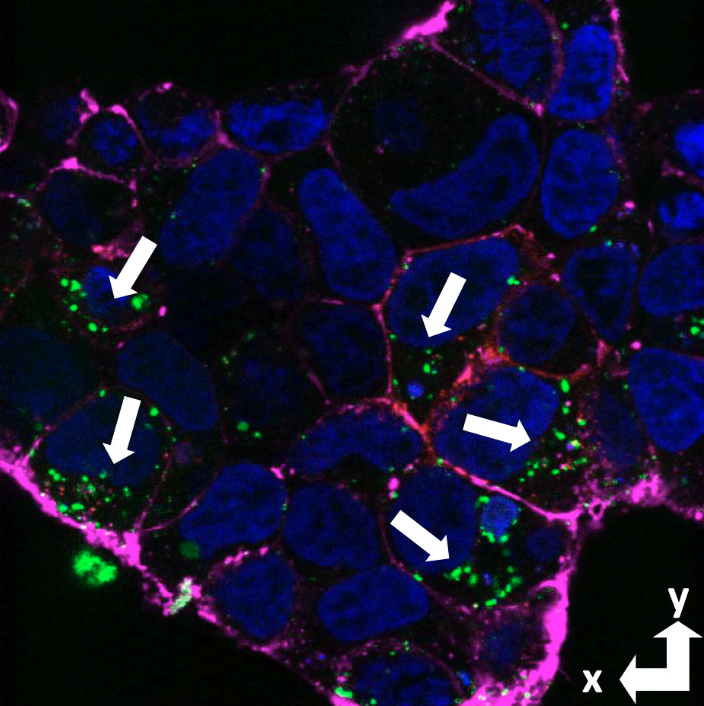
PrPC is
a primary receptor for the Aβ
peptide and its aggregates, which
form senile plaques that initiate
Alzheimer's disease. Confocal
microscopy shows that Aβ with a
fluorescent tag (green) is imported
into PrP expressing cells. (PNAS
117:28625-31
2020).
|
Research Funded by the
National Institute of General Medical Sciences, NIH R35
GM131781
Professor Glenn
L. Millhauser
Department of Chemistry & Biochemistry
University of California, Santa Cruz
Santa Cruz, CA 95064
glennm at ucsc.edu
office phone 831 459 2176
lab phone 831 459 3390
fax 831 459 2935
Web design by Chloe S. Millhauser